Author: Catlin Cox
APOL1 genetic variants explain most of the higher kidney disease risk in people of African ancestry. Learn how these inherited genes work, what they mean for your health, and how to protect your kidneys.
Osteoporosis weakens bones and increases fracture risk. Bisphosphonates are the most common treatment, slowing bone loss and reducing fractures by up to 50%. Learn how they work, their risks, and how they compare to newer drugs.
New York's 2024 patient protection laws stop surprise bills, ban providers from filling out financing apps, and block credit card demands before emergency care. These rules are changing how Americans pay for medical treatment-and setting a national standard.
Eosinophilic esophagitis is a food-triggered allergic condition causing esophageal inflammation. Dairy is the most common trigger, and steroid slurries are an effective medical treatment. Elimination diets and targeted therapies can bring relief.
Light-sensitive medications and eye drops can lose up to 50% of their potency if stored improperly. Learn how to protect them with amber containers, proper temperature control, and safe storage spots - and what signs mean it's time to toss them.
Patch testing is the most reliable way to identify metal and fragrance allergies causing chronic skin rashes. Learn how it works, what it detects, and why it's the only method that gives real answers.
Learn which antihypertensive combination generics are available, how much they cost, and how to get them covered by insurance. Find out when combos make sense-and when separate pills are better.
DRESS syndrome is a rare but life-threatening drug reaction that causes fever, rash, organ damage, and high eosinophil levels. Often misdiagnosed, it requires immediate treatment. Learn the signs, triggers, and how to survive it.
Every U.S. state has laws about when pharmacists can swap brand-name drugs for generics or biosimilars. These rules vary widely-some require your consent, others don’t even tell you. Learn how to protect yourself and understand your rights.
ACE inhibitors help control blood pressure but can raise potassium levels dangerously. Learn which foods to watch, who’s at risk, and how to stay safe without giving up healthy eating.
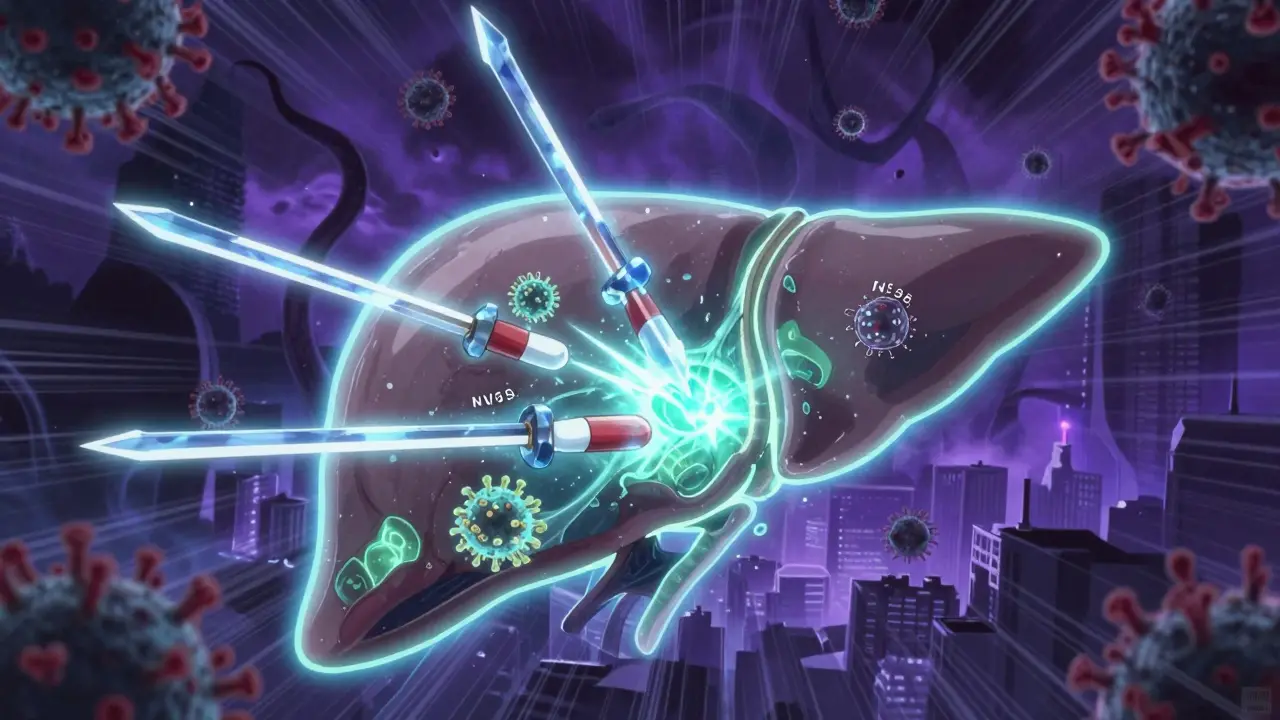
Chronic hepatitis C is now curable in most cases with simple, well-tolerated antiviral pills. These treatments clear the virus, reverse liver damage, and prevent cancer - with cure rates over 95%. Access remains the biggest barrier.
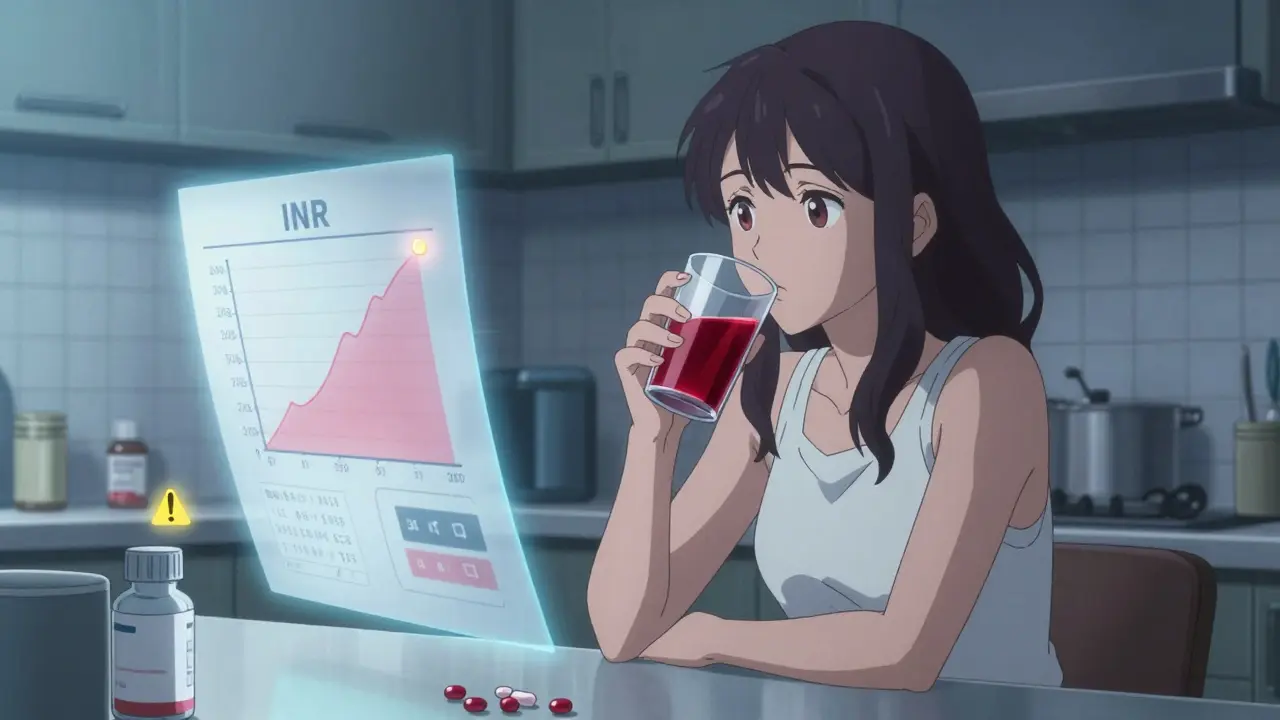
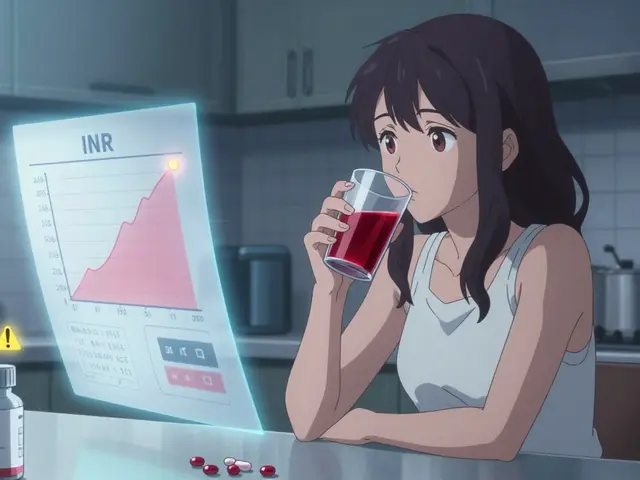
Cranberry juice is safe for most people taking medications, except possibly warfarin. Learn the real risks, debunk the myths, and find out what you actually need to avoid.
Recent-posts
Categories
Tags
- online pharmacy
- side effects
- drug interactions
- generic drugs
- online pharmacy UK
- drug safety
- opioid side effects
- pill organizer
- Tadalafil
- arthritis medication
- buy medication online
- prescription medication
- quit smoking
- motion sickness
- Sildenafil
- Vardenafil
- ED medication alternatives
- biologics
- medication safety
- generic medication prices