When a pharmacist hands you a generic pill instead of the brand-name version, they’re not guessing. They’re relying on one official source: the FDA’s Orange Book. This isn’t just a dusty reference manual-it’s the living, daily-updated database that tells pharmacies exactly which generics can be swapped for brand drugs without risking your health. If you’re a pharmacist, a pharmacy technician, a healthcare provider, or even a patient trying to understand why your prescription changed, knowing how to use the Orange Book correctly matters. A wrong lookup could mean a drug that doesn’t work the same way, or worse, a substitution that’s illegal in your state.
What the FDA Orange Book Actually Is
The FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations, known as the Orange Book, is the only official government source that rates whether a generic drug is truly interchangeable with its brand-name counterpart. It’s not a list of every drug on the market-it’s a targeted tool for therapeutic equivalence. Created under the Hatch-Waxman Act of 1984, it was designed to speed up generic drug approvals while keeping patient safety front and center.Today, it covers over 16,000 approved drug products, including both prescription and some over-the-counter (OTC) medications. But here’s the key: only prescription drugs get therapeutic equivalence ratings. OTC drugs are listed in the Orange Book, but they don’t have TE codes. That’s because the FDA doesn’t evaluate them for substitution. If you’re checking for a generic version of Tylenol or Zyrtec, you won’t find a reliable equivalence rating here.
The Orange Book doesn’t just list drugs-it connects them. Each brand-name drug has a designated Reference Listed Drug (RLD). Every generic that’s been tested and approved to match that RLD gets a Therapeutic Equivalence (TE) code. That code is the gatekeeper for pharmacy substitution.
Understanding Therapeutic Equivalence Codes
The TE code is a two-letter rating that tells you whether a generic is considered interchangeable. It’s simple, but it’s also the most critical part of the whole system.- AB means the generic is therapeutically equivalent to the RLD. It has the same active ingredient, dosage form, strength, and route of administration-and it’s been proven bioequivalent. You can substitute it without hesitation.
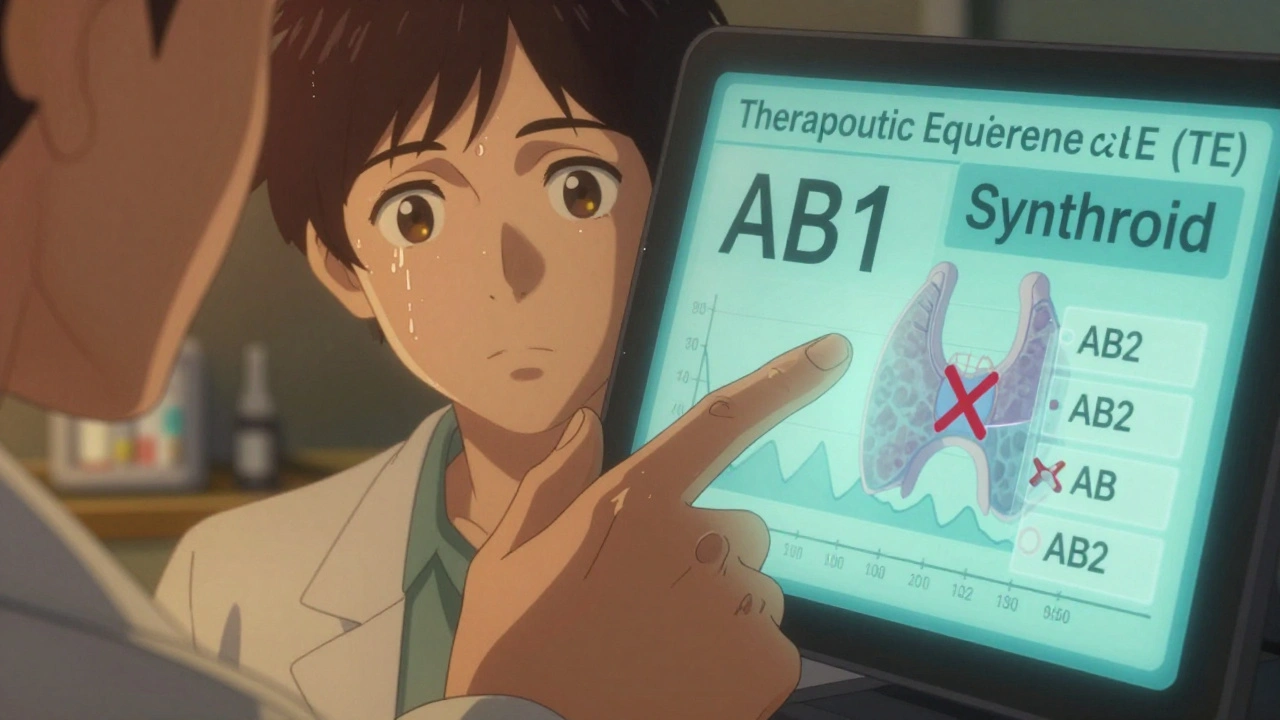
- AB1, AB2, AB3 are variations when there are multiple RLDs for the same active ingredient. For example, if two different brand versions of levothyroxine exist, generics might be rated AB1 for one and AB2 for the other. Mixing them up could mean switching between non-interchangeable versions.
- B means the generic is not considered therapeutically equivalent. This usually happens when bioequivalence data is incomplete, the formulation is different, or there’s a known issue with absorption. These should never be substituted without a doctor’s approval.
- BX is a special case. It means the FDA has insufficient data to rate the product. These are often newer generics or complex formulations like inhalers or topical creams. Treat them like untested drugs.
Here’s what the FDA says: “Products rated AB are expected to have the same clinical effect and safety profile as the RLD when administered under the conditions specified in the labeling.” That’s the standard. But it’s not a guarantee for every patient. Some drugs-like blood thinners, seizure meds, or thyroid hormones-have a narrow therapeutic index. That means even tiny differences in absorption can cause big problems. The Orange Book doesn’t flag these risks. You have to know them yourself.
How to Search the Electronic Orange Book
The FDA stopped printing the Orange Book in 2007. Everything’s online now. Go to the Electronic Orange Book (EOB) on the FDA’s website. It’s free, updated daily, and redesigned for clarity since 2020.Follow these steps every time you verify a generic:
- Start with the brand name. Type the brand drug’s name (like Synthroid or Lipitor) into the search bar. Don’t use the active ingredient yet-start with the brand to find the RLD.
- Look for the RLD. In the results, find the product marked with “Yes” in the RLD column. That’s the original drug the generics are being measured against.
- Filter by dosage form and route. Click on the RLD’s dosage form (e.g., “tablet, oral”) and route (e.g., “oral”). This groups all products with the same delivery method.
- Check the TE Code column. Every generic in that group will show a TE code. Only those with “AB” (or AB1, AB2, etc.) are approved for substitution.
- Verify the applicant. Look at the manufacturer. Some companies make multiple versions of the same drug. Make sure you’re matching the right one.
Pro tip: Use the “Ingredient Search” only after you’ve confirmed the RLD. Searching by active ingredient alone can give you hundreds of results, including discontinued products and OTC drugs. You’ll waste time unless you know what you’re looking for.
What the Orange Book Doesn’t Tell You
The Orange Book is powerful-but it’s not complete. It doesn’t tell you about state laws, insurance rules, or patient-specific risks.For example: Levothyroxine (Synthroid) has dozens of AB-rated generics. But in 45 states, pharmacists can’t substitute them without a doctor’s note. Why? Because thyroid hormones have a narrow therapeutic index. Even a 5% difference in absorption can push a patient into hyper- or hypothyroidism. The Orange Book says “AB”-but your state’s pharmacy board says “no substitution.”
Also, the Orange Book doesn’t track:
- Patent litigation delays that block generic entry
- Drug shortages that force substitutions anyway
- Insurance formulary restrictions that make certain generics cheaper
- Manufacturing changes that happen after approval
That’s why pharmacists still need to use their judgment. The Orange Book tells you what’s legally allowed. It doesn’t tell you what’s safest for your patient.
Common Mistakes and How to Avoid Them
Even experienced professionals make these errors:- Mixing up AB1 and AB2. If a drug has two RLDs, the TE code tells you which one the generic matches. Substituting an AB1 generic for an AB2 RLD could be unsafe. Always check the RLD designation.
- Ignoring discontinued products. The Orange Book has a separate list for drugs no longer on the market. If you see a generic with no TE code, check the Discontinued Drug Product List. It might be off the market.
- Confusing patent dates with exclusivity. A patent expiration doesn’t mean a generic can be sold. Market exclusivity (like 180-day exclusivity for first filers) can delay entry. The Orange Book lists both, but they’re not the same.
- Trusting third-party apps. Drugs.com, Micromedex, and other tools pull data from the Orange Book-but they’re often 24 to 72 hours behind. If you’re verifying for a patient right now, use the FDA site. Always.
The FDA recommends doing at least five verifications to get comfortable. Start with a common drug like atorvastatin (Lipitor) or metformin (Glucophage). Compare the brand to its generics. Look at the TE codes. Check the RLD. Do it again tomorrow. You’ll build confidence fast.
When to Use the Orange Book
You should check the Orange Book in these situations:- A patient asks why their prescription changed from brand to generic.
- A prescriber orders a generic, but you’re unsure if it’s approved for substitution.
- Your pharmacy’s formulary changes and you need to verify new substitutions.
- You’re reviewing a patient’s medication history and spot a switch that doesn’t make sense.
- You’re training a new pharmacy technician or student.
Don’t use it for:
- OTC drugs (they’re not rated)
- Biologics (use the Purple Book instead)
- Compounded medications (they’re not FDA-approved)
- Drugs outside the U.S. (other countries have their own systems)
What Comes Next for the Orange Book
The FDA is working to make the Orange Book smarter. By 2024, it’s expected to integrate with the Purple Book (for biologics), and data will be available in machine-readable formats like SPL (Structured Product Labeling). That means pharmacies and EHR systems will soon pull Orange Book data automatically-no manual search needed.But until then, the manual process is still the gold standard. The FDA offers free training videos, a 12-page Quick Reference Guide, and email support ([email protected]) that responds within 48 hours. Use them. Don’t guess.
At the end of the day, the Orange Book isn’t just a database. It’s the foundation of the U.S. generic drug system. It saves patients billions every year. But it only works if you use it right.
Is the FDA Orange Book free to use?
Yes, the Electronic Orange Book is completely free and publicly accessible on the FDA’s website. No registration, login, or subscription is required. All data is updated daily and maintained by the FDA’s Center for Drug Evaluation and Research.
Can I substitute any generic labeled AB with the brand name?
Legally, yes-if your state allows substitution. But not always safely. Drugs with narrow therapeutic indexes (like warfarin, levothyroxine, or phenytoin) may require a doctor’s approval even if they’re AB-rated. Always check your state’s pharmacy laws and consider patient history before substituting.
Why do some generics have AB1, AB2, or AB3 codes?
These codes appear when a drug has multiple Reference Listed Drugs (RLDs). For example, two different brand versions of the same active ingredient may have been approved at different times. Each generic is tested against one specific RLD. AB1 means it matches RLD #1, AB2 means it matches RLD #2. Substituting a generic with the wrong RLD can lead to ineffective treatment.
Are over-the-counter (OTC) drugs listed in the Orange Book?
Yes, OTC drugs are listed in the Orange Book, but they do not receive therapeutic equivalence (TE) codes. The FDA only evaluates prescription drugs for substitution. If you’re looking for an OTC generic equivalent, you’ll need to check the drug’s active ingredient and compare labels manually.
What should I do if I can’t find a generic in the Orange Book?
If a generic isn’t listed, it may be discontinued, not yet approved, or an OTC product. Check the Discontinued Drug Product List on the FDA site. If it’s a new generic, it may take weeks to appear in the database. Never assume a product is equivalent just because it has the same active ingredient. Always verify through the official Orange Book.







Saket Modi
December 2, 2025 AT 05:40Chelsea Moore
December 3, 2025 AT 19:52John Morrow
December 4, 2025 AT 16:46Kristen Yates
December 6, 2025 AT 04:51Saurabh Tiwari
December 6, 2025 AT 12:21Michael Campbell
December 7, 2025 AT 02:41Victoria Graci
December 8, 2025 AT 07:18Saravanan Sathyanandha
December 10, 2025 AT 01:01alaa ismail
December 10, 2025 AT 15:44ruiqing Jane
December 10, 2025 AT 22:33Fern Marder
December 11, 2025 AT 22:00Carolyn Woodard
December 11, 2025 AT 22:13Allan maniero
December 12, 2025 AT 05:39Anthony Breakspear
December 12, 2025 AT 07:57Zoe Bray
December 13, 2025 AT 21:03