When a patient has a bad reaction to a generic drug, who do you report it to? And how do you even know which company made it? The answer isn’t as simple as it should be. Even though generic drugs make up 90% of all prescriptions filled in the U.S., they account for far fewer serious adverse event (SAE) reports than brand-name drugs. That’s not because generics are safer-it’s because the system isn’t working right.
What Counts as a Serious Adverse Event?
A serious adverse event isn’t just a mild side effect. The FDA defines it as any reaction that’s life-threatening, causes hospitalization, leads to permanent disability, results in a birth defect, or requires medical intervention to prevent lasting harm. These include things like severe allergic reactions, liver failure, heart rhythm problems, or sudden loss of vision. It doesn’t matter if the drug is brand or generic-if it’s serious, it needs to be reported.Both brand and generic drug manufacturers are legally required under 21 CFR 312.64(b) and 21 CFR 310.305 to report these events to the FDA within 15 days. The same rules apply. But here’s the problem: while generics are used in nine out of ten prescriptions, they only show up in about 30% of serious adverse event reports. That gap isn’t random. It’s systemic.
Why Generic Drug Reports Are So Underreported
The issue isn’t that generic drugs are less dangerous. It’s that the reporting system is designed for brand-name products-and it’s not built for the reality of today’s generic market.Take a common drug like losartan. In 2018, a study of over 10 years of FDA data found that even after generic versions became widely available, brand-name reports kept climbing. Meanwhile, reports for generic versions stayed flat. Why? Because most patients don’t know which company made their pill. Pharmacies switch suppliers all the time. One month, it’s Teva. The next, it’s Mylan. The label might say “losartan 50 mg,” but the manufacturer’s name is printed in tiny font, if it’s there at all.
Healthcare providers face the same confusion. A 2020 survey by the Institute for Safe Medication Practices found that 68% of pharmacists and doctors had trouble identifying the specific generic manufacturer when filling out a report. For brand-name drugs, that number was only 12%. In the FDA’s own 2019 usability study, 42% of providers abandoned their reports for generic drugs because they couldn’t figure out who made them.
Even when they try, the process is clunky. The FDA’s MedWatch form asks for the brand name or generic name-and then the manufacturer. But if the patient doesn’t know the manufacturer, and the pharmacist didn’t document it, the provider is stuck. Many end up reporting to the brand-name company by default, which distorts the data and hides real safety signals.
How to Report a Serious Adverse Event from a Generic Drug
If you’re a clinician, pharmacist, or patient and you see a serious reaction linked to a generic drug, here’s how to report it correctly:- Check the medication bottle. Look for the manufacturer name. It’s usually on the side or bottom of the bottle or blister pack. It might say “Teva,” “Aurobindo,” “Amneal,” or “Sandoz.” Don’t assume it’s the same as last time.
- Find the NDC code. The National Drug Code is a 10- or 11-digit number on the label. Write it down. You can look it up later on DailyMed.nih.gov to confirm the manufacturer.
- Use MedWatch Form 3500. Go to the FDA’s MedWatch website. Select “Health Professional” or “Consumer” depending on who’s reporting. Choose “Generic Drug” under product type.
- Enter the exact generic name and manufacturer. Don’t use the brand name unless you’re sure it’s the same product. For example, write “amlodipine besylate” and “Teva Pharmaceuticals USA.”
- Describe the event fully. Include when it started, symptoms, duration, treatment, and outcome. Even if you’re unsure if it’s linked to the drug, report it anyway. The FDA needs all data.
- Submit electronically. Online submission is fastest. You can also fax or mail the form, but delays mean slower response times.
Pro tip: If you’re in a hospital or clinic, ask if your pharmacy uses barcode scanning at the point of dispensing. Hospitals that do this have seen a 63% increase in accurate generic drug reporting, according to ASHP guidelines.

What Happens After You Report
Once the FDA receives your report, it goes into the FAERS database-their main safety monitoring system. These reports are analyzed for patterns. If multiple reports point to the same generic manufacturer and the same reaction, the FDA may investigate further. That could mean updating labels, issuing safety alerts, or even requesting more testing.But here’s the catch: if reports are incomplete or misattributed, the FDA can’t see the signal through the noise. That’s why the agency launched FAERS 2.0 in 2023, which now tracks adverse events by NDC code. That’s a big step forward. It means they can finally tell which specific generic manufacturer is linked to a problem-not just the active ingredient.
The FDA also issued draft guidance in June 2023 asking pharmacies to print the manufacturer name clearly on all prescription labels. If that rule becomes final, it could cut reporting errors by more than half.
The Bigger Problem: Systemic Underinvestment
Brand-name drug companies spend millions on pharmacovigilance teams. They have dedicated staff tracking every side effect, analyzing data, and communicating with regulators. Generic manufacturers? Not so much.A 2022 survey found that 98% of brand-name companies have full-time pharmacovigilance departments. Only 42% of generic manufacturers do. Smaller generic companies often outsource reporting to third parties-leading to delays, lost data, or inconsistent standards. The top 10 generic makers handle 65% of prescriptions, but smaller ones-those making 32% of the supply-filed only 4.7% of adverse event reports.
The FDA’s Generic Drug User Fee Amendments (GDUFA) are starting to change that. GDUFA III, which runs through 2027, includes $15 million specifically for improving generic drug safety monitoring. Industry spending on pharmacovigilance tech is expected to jump from $185 million in 2023 to $320 million by 2027. That’s progress. But it’s still not enough to close the gap overnight.
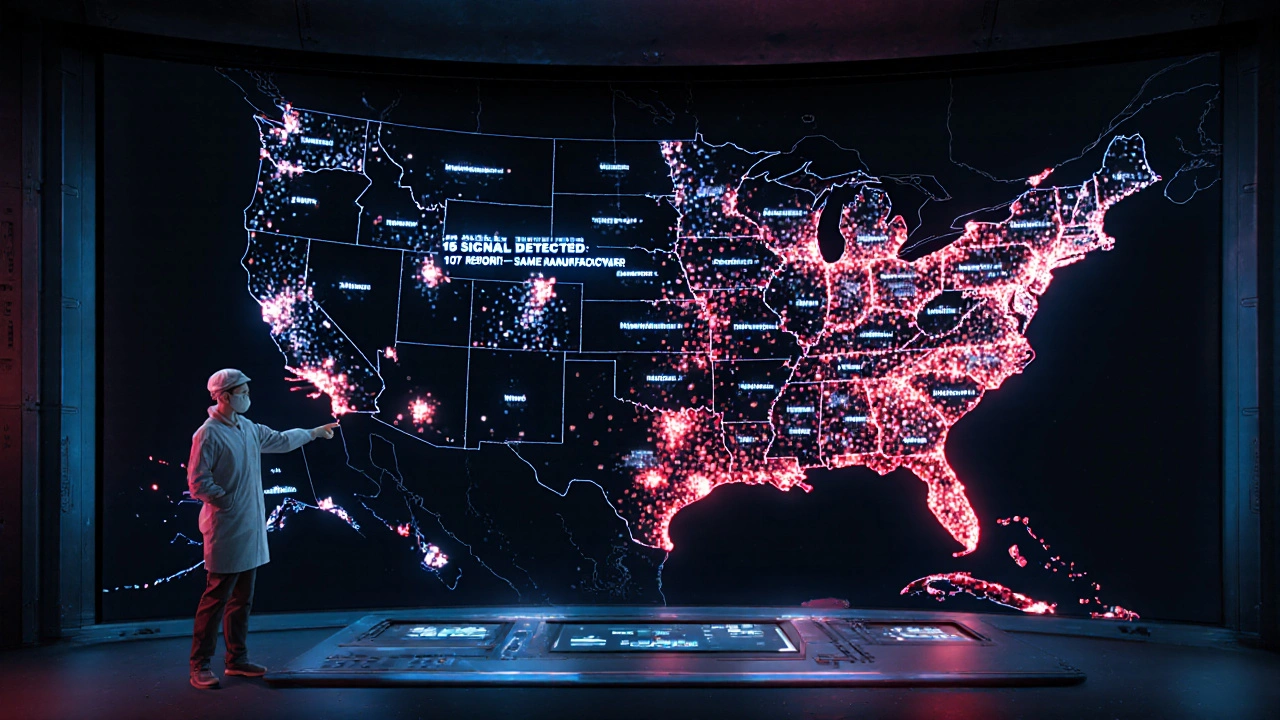
What Patients and Providers Can Do Today
You don’t need to wait for policy changes to make a difference. Here’s what you can do right now:- Patients: Keep your medication bottles until you finish the prescription. Take a photo of the label if you’re worried about switching. Write down the manufacturer name if you notice a new reaction.
- Pharmacists: If you’re dispensing a generic, write the manufacturer name on the prescription slip or patient instructions. Make it part of your workflow.
- Doctors: Ask patients, “Do you know which company made your pill?” If they say no, help them find out. Don’t assume it’s the same as last time.
- All providers: Report every serious reaction-even if you’re unsure. The FDA needs more data, not less.
Every report matters. One report might seem small. But if 100 people report the same reaction to the same generic version of a drug, that’s a signal the FDA can’t ignore.
The Future of Generic Drug Safety
The FDA’s 2024 pilot program with major pharmacy chains to automatically capture manufacturer data at the point of dispensing could be a game-changer. Early modeling shows it could boost reporting completeness by 55% in three years. If it works, it could become national policy.Meanwhile, the European Medicines Agency is pushing for similar changes. Their 2022-2025 pharmacovigilance plan lists “improving signal detection for generic medicines” as a top priority. This isn’t just a U.S. problem-it’s a global one.
Without action, the Congressional Budget Office warns that by 2030, we could miss 15 to 20 unsafe generic drugs every year because the data is too fragmented to detect patterns. That’s not just a data gap. It’s a patient safety risk.
Generic drugs save the U.S. healthcare system over $300 billion a year. They’re essential. But saving money shouldn’t mean sacrificing safety. Reporting serious adverse events for generic drugs isn’t optional-it’s a critical part of keeping everyone safe.
Do I have to report adverse events for generic drugs even if I’m not sure they caused the problem?
Yes. The FDA asks for all serious adverse events that may be linked to a drug, even if the connection isn’t certain. Your report helps build the big picture. If multiple people report the same reaction after taking the same generic drug, it becomes a signal worth investigating. Don’t wait for proof-report early.
Can I report an adverse event if I’m a patient, not a healthcare provider?
Absolutely. Patients can and should report serious reactions directly to the FDA using MedWatch Form 3500. You don’t need a medical license. Just go to the FDA’s MedWatch website, select “Consumer,” and fill out the form. Include the drug name, manufacturer (if known), your symptoms, and when they started.
Why does the manufacturer name matter so much in reporting?
Different generic manufacturers use different inactive ingredients-fillers, dyes, coatings-that can affect how a drug is absorbed. One manufacturer’s levothyroxine might work perfectly for you, but another’s could cause symptoms because of a different binder. If you don’t report the manufacturer, the FDA can’t tell which version is causing the issue. That’s how unsafe batches go unnoticed.
What if the pharmacy won’t tell me who made my generic drug?
Check the bottle label-it should have the manufacturer’s name. If it doesn’t, call the pharmacy and ask for the National Drug Code (NDC) number. Then look it up on DailyMed.nih.gov. That site shows you the exact manufacturer and product details tied to each NDC. You don’t need to guess.
Is there a faster way to report generic drug reactions?
Yes. Many hospitals and clinics now use electronic health record systems that integrate with MedWatch. If you’re in one of those systems, you can often click a button to auto-fill the drug name and NDC code from your prescription record. Ask your pharmacy or IT department if this feature is available. It cuts reporting time from 30 minutes to under 5.
What’s being done to fix the reporting gap for generic drugs?
The FDA is rolling out FAERS 2.0, which tracks adverse events by NDC code so they can link reactions to specific manufacturers. They’re also pushing pharmacies to print manufacturer names clearly on labels. A 2024 pilot with major pharmacy chains aims to automatically capture that info at dispensing. Industry spending on pharmacovigilance tech is rising, and GDUFA III is funding new safety tools. Progress is happening-but it needs more public participation to work.
What Comes Next
If you’ve ever wondered why a generic drug suddenly started causing problems for multiple patients, now you know: it’s not always the drug itself. It’s the system around it. The same active ingredient can behave differently depending on who made it and how it was formulated. Reporting isn’t just paperwork-it’s the first line of defense.Next time you fill a prescription for a generic drug, take a second to check the label. If you see a reaction, don’t ignore it. Don’t assume someone else will report it. Your report could be the one that stops a dangerous pattern before it hurts more people.









Holly Powell
November 19, 2025 AT 00:57The systemic underreporting of generic drug adverse events is a pharmacovigilance catastrophe masked as bureaucratic inertia. The FDA’s reliance on voluntary, manufacturer-agnostic reporting frameworks is archaic. NDC-level granularity isn’t optional-it’s the minimum viable data point for signal detection. Until every generic product is tied to a unique, machine-readable identifier at point-of-dispense, we’re just noise-filtering in the dark. The 68% pharmacist confusion rate? That’s not a gap-it’s a systemic failure of supply chain traceability. GDUFA III funding is a Band-Aid on a hemorrhage.
Emanuel Jalba
November 20, 2025 AT 11:01THIS IS WHY PEOPLE DIE 😭 I filled my losartan for 3 months and never knew it switched from Teva to Aurobindo-then I got liver enzymes through the roof. No one told me. No one asked. And now I’m stuck with a $12k hospital bill because the system is BROKEN. 🚨 #GenericDrugNightmare
Heidi R
November 22, 2025 AT 00:14Bailey Sheppard
November 23, 2025 AT 09:56Really appreciate this breakdown. I’m a pharmacist and I’ve seen the confusion firsthand-patients come in with a bottle that says ‘amlodipine’ and no manufacturer. We’re not trained to dig into NDC codes unless there’s a problem. Maybe we need a sticker on every generic bottle that says: ‘Made by: [NAME] - Report issues here: fda.gov/medwatch’? Simple, clear, saves lives.
Shilpi Tiwari
November 24, 2025 AT 01:42As someone from India where 80% of global generics are manufactured, this resonates deeply. The supply chain is a labyrinth-Teva might source from a facility in Hyderabad, which is owned by a subsidiary of a Swiss holding company, which outsources labeling to a third-party vendor in Manila. The FDA’s FAERS 2.0 is a step forward, but global harmonization of NDC-like codes is the real solution. We need ISO 11615 for generics, not just U.S.-centric fixes.
Christine Eslinger
November 24, 2025 AT 06:44One of the most important things I’ve read all year. I used to think ‘it’s just a generic’-until my mom had a severe reaction to a generic metoprolol that wasn’t her usual brand. We didn’t know until we dug into the bottle and found ‘Zydus’ instead of ‘Lupin’. That’s when I realized: the active ingredient is the same, but the delivery system isn’t. The FDA’s new label requirements? Long overdue. But now it’s on all of us to act. Take a photo. Write it down. Report it. One report might be the one that changes the system.
Denny Sucipto
November 25, 2025 AT 03:27Man, I’ve been a nurse for 18 years and I never thought twice about which company made the generic. But after my buddy had a seizure on a generic gabapentin and we found out it was made by a company that got a warning letter from the FDA last year? That changed everything. Now I make my patients show me the bottle before they leave. I even keep a little cheat sheet on my desk with the top 20 generic makers and their common red flags. Small thing. Big difference.
Kristi Joy
November 26, 2025 AT 05:56I want to thank you for writing this. It’s easy to feel powerless when you’re just a patient or a frontline provider. But you’re right-every report matters. I’ve started keeping a log in my phone: drug name, manufacturer, reaction, date. I don’t report every little thing, but if it’s serious, I file it. I’ve filed three so far. Two got flagged by the FDA. One led to a recall. You don’t have to be a doctor to be a watchdog. You just have to care enough to speak up.
Hal Nicholas
November 26, 2025 AT 10:29Let’s be real: this isn’t about safety. It’s about profit. Big Pharma wants you to think generics are safe so they can keep charging $1000 for their brand-name drugs. The FDA? They’re cozy with the big generic manufacturers who donate to their lobbying groups. FAERS 2.0? A PR stunt. The real solution? Ban generics. Go back to brand-only. Then we’ll see how fast the system fixes itself.
Louie Amour
November 27, 2025 AT 17:30Wow. Just wow. You people are naive. The FDA doesn’t care about your ‘reports.’ They’re funded by industry user fees. GDUFA? That’s the Generic Drug User Fee Act-meaning the manufacturers pay the FDA to regulate themselves. So yes, the system is rigged. You think they’re going to investigate a $0.05 pill made by a company that pays their salary? Wake up. This isn’t a reporting problem. It’s a corruption problem. Stop feeding the machine.
Kristina Williams
November 28, 2025 AT 05:02They’re hiding something. Why do ALL the bad reactions happen after the generic switch? Why do the same 3 companies always show up in the reports? Why do the labels change every 3 months? This isn’t coincidence. It’s deliberate. The Chinese government owns half the generic manufacturers. They’re testing new compounds on Americans. Your meds are being used as a bio-warfare experiment. And they don’t want you to know.
Brenda Kuter
November 29, 2025 AT 01:23I’ve been waiting for someone to say this. I work at a pharmacy and we’re told not to mention the manufacturer unless asked. Why? Because if patients find out their drug switched from Teva to Aurobindo, they panic. They call the doctor. They file complaints. So we keep quiet. We’re complicit. And now my cousin is in the ICU because her generic levothyroxine had a different filler. She didn’t know. We didn’t tell her. We’re monsters.
Shaun Barratt
November 29, 2025 AT 21:54While the intent of this exposition is commendable, the structural deficiencies in pharmacovigilance infrastructure merit more rigorous epistemological scrutiny. The NDC code, while technically adequate, remains insufficiently granular for causal attribution in polypharmacological contexts. A blockchain-based, cryptographically signed, immutable ledger-linked to batch-level manufacturing data and dispensed via QR code at point-of-sale-is the only viable solution. The current system is epistemologically bankrupt. We must transition to a decentralized, auditable, real-time pharmacovigilance protocol immediately.