When your regular medication runs out and no pharmacy has it in stock, it’s not just inconvenient-it’s dangerous. In 2025, over 1,961 prescription drugs were in short supply across the U.S. and parts of Europe, with many shortages lasting more than two years. Insulin, antibiotics like amoxicillin, chemotherapy agents, and even basic pain relievers like acetaminophen injections have all hit critical lows. You can’t just wait it out. If you’re on a medication that’s disappeared from shelves, here’s exactly what to do-step by step.
Check the FDA Drug Shortage Database First
The first thing you should do is go to the FDA Drug Shortage Database. It’s free, updated daily, and lists every active shortage with the reason and possible alternatives. Don’t rely on your pharmacist alone-many don’t have real-time inventory data. The FDA database tells you if a shortage is temporary or long-term, and often lists therapeutically equivalent substitutes. For example, when Semglee (a biosimilar insulin) ran out in March 2025, the FDA flagged Lantus as a direct replacement. No new prescription needed. That’s critical info you won’t get from a voicemail at your pharmacy.Don’t Guess-Talk to Your Doctor About Alternatives
Switching medications isn’t something to do on your own. Even if two drugs seem similar, they can have different side effects, dosing schedules, or interactions. During the amoxicillin shortage, some doctors switched patients to azithromycin. But that’s not always safe-azithromycin increases the risk of heart rhythm issues in older adults and can worsen antibiotic resistance. Your doctor knows your full history: other meds you take, allergies, kidney or liver function. They can pick a true alternative, not just a convenient one. In fact, 68% of patients who successfully switched during shortages did so only after a detailed conversation with their provider, according to the Sterling Institute’s 2025 survey.Ask Your Pharmacist About Biosimilars and Formulary Changes
Many shortages involve brand-name drugs, but biosimilars-medications that are nearly identical in effect-are often still available. Semglee and Lantus are a perfect example: they’re both insulin glargine, and the FDA says they’re interchangeable. That means your pharmacist can swap them without a new prescription. But not all pharmacies know this. Ask specifically: “Is there a biosimilar or formulary-approved substitute for this drug?” Blue Cross NC changed its rules in March 2025 to allow pharmacists to switch patients from Semglee to Lantus automatically. If your insurer changed coverage rules, your pharmacist should know. Call ahead. Most major chains now have shortage specialists on staff-89% offer free consultations just for this purpose.Try Mail-Order Pharmacies and Regional Chains
Big chain pharmacies like CVS or Walgreens often run out at the same time because they buy from the same distributors. But mail-order pharmacies and regional chains have different supply chains. One patient in Chester spent three days calling seven local pharmacies for Semglee-none had it. Then they tried a mail-order service through their insurance plan. It arrived in four days. Mail-order isn’t faster because it’s “better”-it’s because it orders in bulk and gets shipments directly from manufacturers. Some regional chains like Rite Aid or independent pharmacies also get stock from smaller suppliers. Ask your pharmacist for a list of nearby pharmacies that might have it. Don’t assume they all have the same inventory.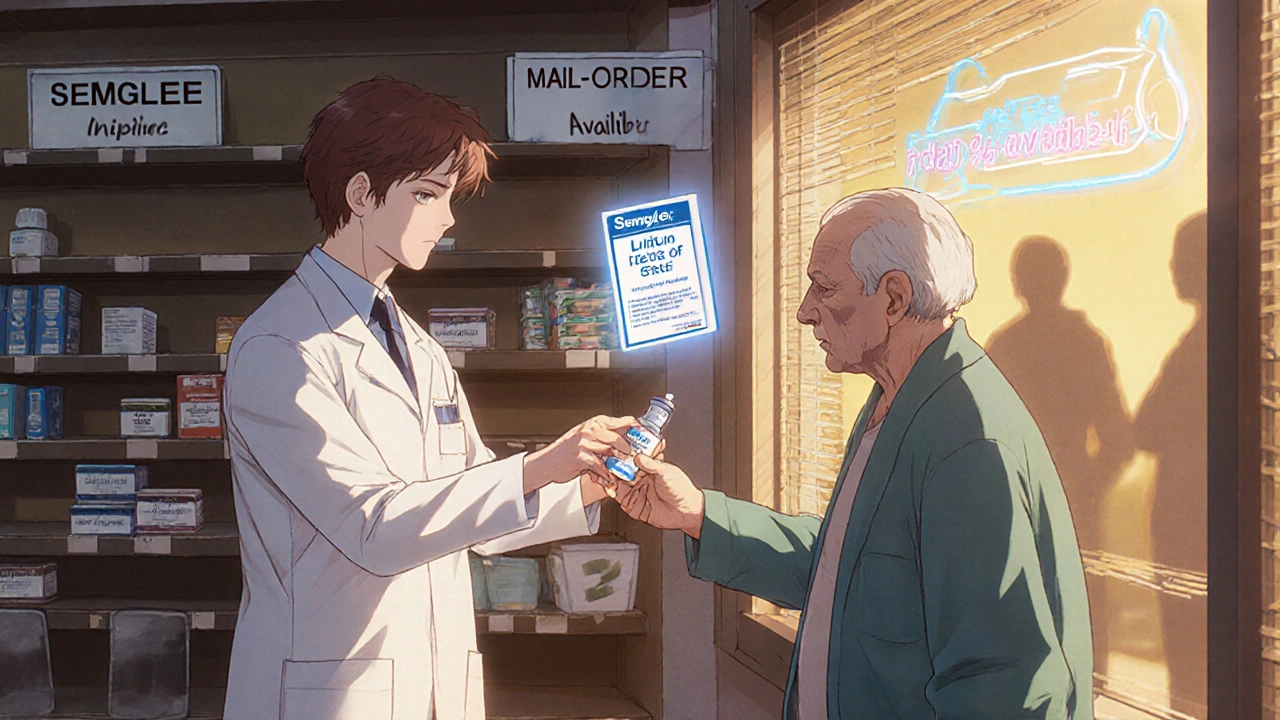
Know When You Can Use Foreign-Approved Drugs
This isn’t a loophole-it’s a legal option in some places. In 2025, Hawaii’s Medicaid program started allowing pharmacists to dispense drugs approved in Canada, the UK, or the EU if the U.S. version is unavailable. These aren’t knockoffs-they’re the same pills made in the same factories, just sold under different names. For example, insulin made in Germany or the UK is chemically identical to U.S. versions. You need a prescription, and your doctor must agree, but it’s a real lifeline. The FDA has a special waiver process for this under Section 804 of the Food, Drug, and Cosmetic Act. Ask your doctor if this is an option for you. It’s not available everywhere yet, but it’s growing.Call the Manufacturer-They’ll Tell You When It’s Coming Back
Manufacturers know exactly when production will resume. Pfizer, for example, gave out exact dates during the amoxicillin shortage: “We’ll have 500,000 doses available by November 15.” That helped patients plan. You can find manufacturer contact info on the FDA’s shortage page or just Google the drug name + “company contact.” Don’t be shy-this is why they have customer service lines. Some even offer free samples or temporary supplies during shortages. If you’re on a chronic medication, ask if they have a patient assistance program. Many do.Never Stop Your Medication Without a Plan
Thirty-two percent of patients in the Sterling Institute survey stopped taking their medication during a shortage. Some thought they could “skip a few days.” Others couldn’t afford the alternative. But stopping insulin, blood pressure meds, or seizure drugs-even for a few days-can lead to hospitalization. If you can’t get your drug, don’t stop. Call your doctor. Ask for a temporary dose reduction or a different form (like a pill instead of injection). If you’re on a drug like alteplase for clot-busting, even small changes in timing can affect outcomes. There’s always a safer path than quitting cold turkey.
Watch for State-Level Help Programs
Some states are stepping in. New Jersey proposed letting pharmacists hand out emergency insulin supplies without a prescription during shortages. California, New York, and Washington are stockpiling abortion medications in case federal restrictions cause supply cuts. In your state, check with your board of pharmacy or state health department. You might qualify for emergency access programs. If you’re on Medicaid or Medicare, ask if your plan has a shortage override policy. Some insurers now allow early refills or higher quantities during shortages.Prepare for the Long Haul
Most shortages aren’t going away. The FDA says 75% of current shortages have lasted over a year. That means you need a long-term plan. Keep a printed list of your medications, dosages, and alternatives your doctor approved. Save contact info for your pharmacist and manufacturer. Talk to your doctor now-not when you’re out of pills-about backup options. If you’re on a drug like sarilumab for autoimmune disease, you might need to adjust your dose. Dutch data shows patients cut their total yearly dose by 67% during shortages, and still stayed stable. It’s not ideal, but it’s safer than stopping.What to Do Right Now
- Go to the FDA Drug Shortage Database and search your medication.
- Call your pharmacy and ask if they have a biosimilar or formulary alternative.
- Ask your doctor: “Is there a safe substitute?” Don’t accept “I don’t know.”
- Try a mail-order pharmacy or regional chain.
- Call the drug manufacturer for a timeline and possible sample program.
- Check your state’s board of pharmacy website for emergency access rules.
Medication shortages are a system problem-but you don’t have to be a victim of it. With the right steps, you can keep taking what you need, safely and without panic.
What should I do if my insulin is out of stock?
First, check the FDA’s drug shortage page to confirm the shortage. Then ask your pharmacist if Lantus or another insulin glargine product is available. Semglee and Lantus are interchangeable biosimilars, so your pharmacist can switch them without a new prescription. If that doesn’t work, call your doctor to discuss alternatives like Toujeo or Tresiba, but be aware those require new prescriptions. Mail-order pharmacies often have better stock than local stores.
Can I use a drug approved in another country?
Yes, in some cases. Hawaii’s Medicaid program and a few other states allow pharmacists to dispense foreign-approved versions of U.S. drugs during shortages. These are the same pills made in the same factories-just labeled differently. You need a prescription and your doctor’s approval. The FDA allows this under Section 804 waivers. It’s not legal everywhere yet, but it’s becoming more common. Ask your pharmacist or state board of pharmacy if it’s an option in your area.
Why do drug shortages keep happening?
Most shortages come from manufacturing problems-contamination, equipment failure, or raw material delays. Eighty-five percent of generic drugs are made by just five companies, so if one factory shuts down, half the market loses supply. The FDA has increased inspections to monthly visits since January 2025, which has reduced new shortages by 15%. But without more manufacturers and better supply chain tracking, these problems will keep coming.
Are there any free resources to track drug shortages?
Yes. The FDA’s Drug Shortage Database is free and updated daily. ASHP also maintains a public list of current shortages with start and end dates. Both are reliable and don’t require registration. Avoid third-party apps that charge for alerts-they often pull data from these same sources anyway.
What if my insurance won’t cover the alternative drug?
Call your insurer’s customer service and ask for a formulary exception. Explain you’re affected by a confirmed drug shortage and need the alternative for medical necessity. Many insurers have special override codes for shortages. Blue Cross NC removed prior authorization for Lantus during the Semglee shortage. If they say no, ask your doctor to write a letter of medical necessity. You’re not alone-this happens often, and insurers usually approve it when backed by FDA data.



Meghan Rose
November 8, 2025 AT 19:40I know this sounds crazy but I literally called 12 pharmacies in my county last week when my amoxicillin ran out. None had it. Then I found a little independent pharmacy 20 minutes away that got a shipment from a regional distributor. They didn't even know they had it until I asked. Seriously, stop assuming all pharmacies are the same. They're not. And yes, I did call the manufacturer-Pfizer gave me a 3-day supply free because they knew I was diabetic and on insulin too. Don't wait till you're desperate.
Steve Phillips
November 8, 2025 AT 20:40Ohhhhh, so let me get this straight-you’re telling me the FDA has a database… and it’s… FREE?!?!? And we’ve been living in the stone age of pharmacy voicemail hell for YEARS?!?!? I’m not mad-I’m just… disappointed in humanity. Like, who decided that the most critical public health crisis of the decade should be solved by… googling? And why does it feel like we’re all just… stumbling through this like blindfolded toddlers?!?!? I’m crying. I’m crying because I’m 47 and I just learned that ‘biosimilar’ isn’t a swear word. I’m sorry, I’m sorry, I’m sorry.
Rachel Puno
November 9, 2025 AT 07:44You got this. Seriously. I’ve been through two insulin shortages and I know how scary it feels. But you’re already ahead just by reading this. Start with the FDA page-it’s simple. Then call your doc and say ‘I need a backup plan, not just hope.’ Most doctors want to help, they just need you to ask. And if you’re nervous calling a pharmacy? Write down what you want to say first. I did. It helped. You’re not alone. Keep going-one step at a time.
Clyde Verdin Jr
November 10, 2025 AT 17:48LOL. So we’re supposed to trust the FDA database? 😂 The same agency that let 37,000 people die from contaminated heparin in 2008? And now you want me to rely on ‘biosimilars’? Please. I’ve seen the lab reports. They’re not ‘interchangeable’-they’re ‘close enough for government work.’ And don’t even get me started on ‘foreign-approved’ drugs. You think Canada’s pharmaceuticals are better? HA. Their factories are just cheaper. I’d rather risk death than take a pill stamped ‘Made in India’ with a British label. 🤡
Key Davis
November 11, 2025 AT 07:12Allow me to offer a formal observation: the structural fragility of the pharmaceutical supply chain, as elucidated in this post, reflects a broader systemic failure in healthcare logistics and regulatory coordination. The FDA database, while commendable, remains a reactive rather than proactive tool. The proliferation of biosimilars is a necessary innovation, yet its implementation is hampered by inconsistent payer policies and pharmacist education gaps. I respectfully urge all stakeholders-patients, providers, insurers, and regulators-to institutionalize standardized shortage protocols at the state level, as exemplified by New Jersey’s emergency insulin initiative. Preparedness, not panic, must be the new standard.
Cris Ceceris
November 11, 2025 AT 08:04I’ve been thinking about this a lot lately. Like… why do we even have this system where your life depends on a pill that might not exist next week? It’s not just about insulin or antibiotics. It’s about how we value health. We treat it like a commodity, not a right. And the fact that we have to Google our way out of a medical emergency says something terrifying about how broken this is. I don’t have answers. But I’m starting to think the real problem isn’t the shortage-it’s that we’ve stopped expecting better.
Brad Seymour
November 12, 2025 AT 17:59Actually, I just got back from the UK last month and I bought my metformin from a pharmacy in Manchester-same batch, same factory as the US version, just cheaper. The pharmacist laughed and said, ‘You Americans always overpay.’ I showed him your post and he said, ‘We’ve been doing this since 2019.’ Honestly? I’m not trying to be a contrarian. But if you’re willing to travel or order online, you can beat this system. It’s not magic. It’s just… not being afraid to look outside the box.
Malia Blom
November 14, 2025 AT 16:03So let me get this straight-you’re telling me the answer to a national health crisis is… calling your pharmacist and hoping they remember that one time they heard about a biosimilar? And the FDA database is the hero? That’s not a solution. That’s a bandage on a hemorrhage. We’re not supposed to be doing this. We’re not supposed to be playing detective just to stay alive. This isn’t resilience. This is a failure of imagination. And if you think ‘mail-order pharmacies’ are the answer, you’re not seeing the big picture. We need to dismantle the monopoly, not navigate it.